COVID-19
testing solution
Vaccination Software and Site Management
- Covered by FEMA Public Assistance.
- Able to process 20-25 PATIENTS per VACCINATOR per HOUR
- Record speed set up time.
- Able to integrate with any State’s Legacy System
- Able to penetrate underserved communities.
COVID19
Turn Key Solutions
Quality Medical Supplies
In North America, South America and the Caribbean
Personal Protective Equipment
OFFERINGS
Covid-19 Testing
Complete end-to-end service from setup to results
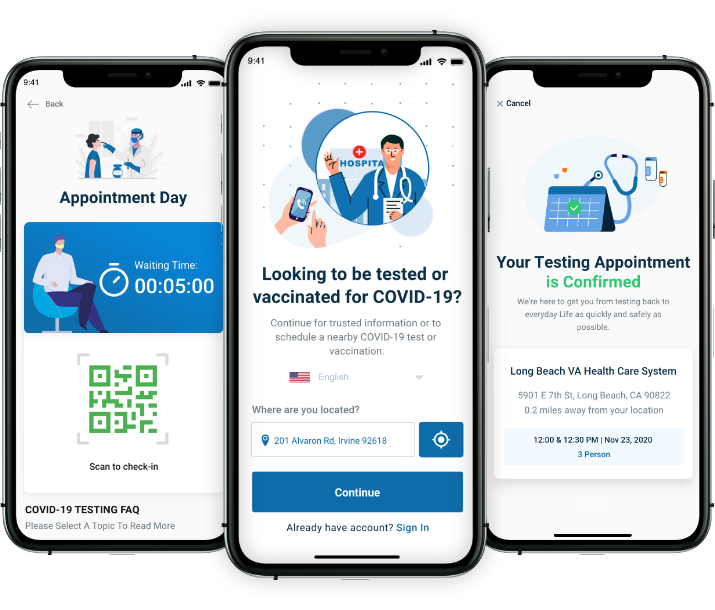
COVID-19 Testing and Vaccination
Platform Solution
Vaccinations are made efficient, easy to access, and scaleable across millions of people. It enables YOU to control, coordinate and follow the vaccination process through an application-based system.
COVID-19
Turn-Key Solutions
Complete end-to-end service from setup to results

School Testing
We want to help schools and higher education safely return to in-person learning. We provide everything you need to setup and run your campus surveillance testing program

Workforce Testing
We provide a full-service testing and program management solution tailored to meet your unique requirements.

State and Local Testing
Protect Your Community
We want to help local communities and states to safely re-open through our massive testing program and vaccination scheduling.

Our medical products bridge the gap between the constant need for quality and the increasing demand for savings.

Our quality system is focused on improving all products and complying with regulatory requirements.

Our product line consists of high quality and high demand supplies distributed throughout Latin America.
About Us
Ariss Medical was created in 2001 with the vision to serve North America, South America and the Caribbean markets.
We represent and distribute quality products manufactured in the United States, Europe, and Asia. Our offices have a 10,800 Sq. ft. warehouse which allows us to constantly rotate products in addition to a staff renown medical advisors who endorse the brands we represent.
Our subsidiary companies are located in Ecuador, Venezuela, Dominican Republic, and the Caribbean Islands.
Our product line consists of high quality and high demand medical supplies distributed throughout North America, South America and the Caribbean Islands.
Featured Products

Mask KN95
The KN95 particulate respirator is designed to help provide protection against non-oil based particles.
5 units
$12.00
Medical 3PLY
Mask
Nonwoven 3-layer Design Spunbond + Meltblown + Spunbond with Ultra-Sonic Weld.
50 pieces per box
$25.00

Daily Protective 3PLY Mask
Nonwoven 3-layer Design Spunbond + Meltblown + Spunbond with Ultra-Sonic Weld.
50 pieces per box
$22.00

Face
Shield
Elastic band to fit all sizes and soft sponge to increase comfort. Anti-fog & anti-static. Protective Film to Avoid Scratch.
1 unit
$6.00

Pandemic Pack
Non-Sterile
AAMI Level 2 Isolation Gown. Disposable 3ply Daily Protective Mask. Disposable Non-Woven Bouffant Cap.
25 units per box
$149.99

Hand Sanitizer 250 ML
Designed to kill a broad-spectrum of pathogenic microorganisms quickly.
1 unit
$3.15

Hand Sanitizer 488 ML
Designed to kill a broad-spectrum of pathogenic microorganisms quickly.
1 unit
$4.35

Clean Room Sticky Mat
Used to remove the dust of shoes. Fast and convenient way of removing contaminants. High stickiness and durability.
Contains 4 packs of 30 sheets
$34.95
Full Distribution Service
-
Sourcing
We partner with top manufacturing companies from USA, Europe and Asia.
-
Import/Export
Our logistics departments handles all imports and exports operations for a hassle-free transaction.
-
Storage
We offer several warehouse facilities in the US, South America, and the Caribbean.
-
Sorting
Products will be repacked in order to fulfill all types of order sizes.
-
Financing
We provide convenient financing services.
-
Market Feedback
Our annalists provide market data and analysis to aid decision making.
It is rare nowadays to get so much quality for the price. Truly good work guys. You should be proud of your range of products. You won me over as a lifelong customer.
Carolina Padron
USA
Your sales support and customer service are far above everyone else we have worked with in the industry. Your customer service representatives are truly knowledgeable about the product and the medical field.
Paolo Espinoza
Ecuador
Our
Distribution Center

Brands We Work With